Empirical Formula and Molecular Formula
Basically the mass of the empirical formula can be computed by dividing the molar mass of the compound by it. Now we will place the number of each atom as a subscript to form the formula.

Unit 6 Molecular And Empirical Formula Sheet Chemistry Classroom Chemistry Lessons Chemistry 101
To find the molecular formula of the Urea First we have to count the number of each atom in the Urea.

. Web The molecular formula describes the composition of ethanol molecules two carbon atoms six hydrogen atoms and one atom of oxygen occur per molecule but gives us no structural information. Multiply every atom subscripts by this ratio to compute the molecular formula. This may or may not be the compounds molecular formula as well.
In some cases the empirical formula is the same as the molecular formula which gives the actual number of atoms in a compound eg H 2 O. As the empirical formula shows the simplest ratio of atoms and molecular formulas display the actual number of atoms in a molecule and structural formulas show how the atoms are bonded to each other. Answers for the test appear after the final question.
This 10-question practice test deals with finding empirical formulas of chemical compounds. From the given molecular formula of sodium hydrogen carbonate one can separate the compound into two ionic components. The compound is formed of a sodium cation Na and an ammonium anion HCO 3-.
A compound contains 8879 oxygen O and. According to the newest conventions effective as of the 20 th May 2019 the mole definition is that a mole is the amount of a chemical substance that contains exactly 602214076 10 23 particles such as atoms molecules ions etc. That is CH 4 N 2 O.
Web Urea Molecular Formula. Web Formula definition a set form of words as for stating or declaring something definitely or authoritatively for indicating procedure to be followed or for prescribed use on some ceremonial occasion. And the molecular formula for benzene which is now going to give us more information than the empirical formula tells us that each benzene molecule has six hydrogens and sorry six carbons and six laughs Im really having trouble today six hydrogens laughs six carbons and six hydrogens.
A simple example of this concept is that the empirical formula of sulfur monoxide or SO would simply be SO as is the empirical formula of disulfur dioxide S 2 O 2Thus sulfur monoxide and disulfur dioxide both compounds. A periodic table will be required to complete this practice test. However additional information is needed to make that determination as discussed later in this section.
Web What is a molecular formula vs empirical formula. Identify which factor is greatest in value. Web The empirical formula for this compound is thus CH 2.
Fe 3 O 2 H 7 2. Consider as another example a sample of compound determined to contain 531 g Cl and 840 g O. Web The empirical formula and molecular formula are related by a whole number ratio.
This is the Molecular Formula of the Urea. Ethyl Alcohol Structural Formula. Web The empirical formula is also known as the simplest formula in chemistry.
Write the factors of each subscript within your formula. Structural representations give even more information than molecular formulas. Structural information is how the atoms are connected and how the molecule fills the space.
Web This chemistry video tutorial explains how to find the empirical formula given the mass in grams or from the percent composition of each element in a compoun. Now the ratio is still. In addition to showing how many of each atom is present in a molecule structural representations give you information about the bonding.
Find the greatest common factor between the subscripts. It gives the smallest whole number ratio of elements in a compound using subscripts following element symbols. Web The empirical formula of a compound represents the simplest whole-number ratio between the elements that make up the compound.
Web To find the ratio between the molecular formula and the empirical formula. Web Sodium hydrogen carbonate chemical formula or mistakenly written as sodium hydroxide carbonate formula is given as NaHCO 3. Web On the other hand if the subscripts do not all share a common factor the molecular formula is also the empirical formula.
Web In chemistry the empirical formula of a chemical compound is the simplest whole number ratio of atoms present in a compound. Web This should be done by providing the empirical chemical formula of the compound involved.

Determining Empirical And Molecular Formulas Chemistry Tutorial Youtube Chemistry Education Chemistry Molecular
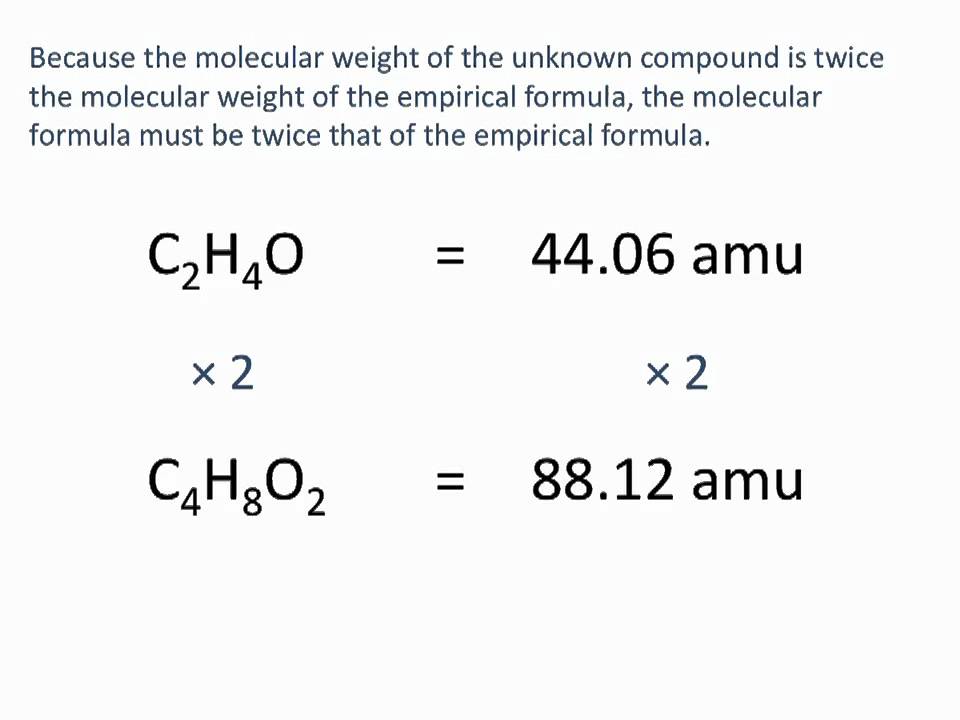
Determining Empirical And Molecular Formulas Chemistry Tutorial Youtube Chemistry Education Chemistry Molecular

Empirical Vs Molecular Formula Molecular Chemistry Education Chemistry Basics

What Is Empirical And Molecular Formula A Plus Topper Https Www Aplustopper Com Empirical And Molecular Formula Emp Chemistry Basics Molecular Formula

Empirical And Molecular Formula Notes Chemistry Worksheets Teaching Chemistry Chemistry Notes

Pin By Red Roses On Study Topic Chemistry Lessons Teaching Chemistry Chemistry Education
Comments
Post a Comment